Target Product Profiles
The beginning is the most important part of the work.
— Plato
The Challenge
Many unmet needs exist and yet, technologies will not be developed to address these gaps due to market shortcomings
Funding organizations and industry representatives are often hesitant to pursue research and development without clear information on product needs. There is a large amount of untapped benefit that could be gained from earlier and more frequent use of mechanisms such as Target product profiles (TPPs). TPPs have traditionally been used in global health, and combined with market-shaping interventions such as Advanced Market Commitments or milestone payments. That’s because the private sector may not invest in diagnostics, therapies, medical devices and vaccines for people living on $2/day in the absence of a government or philanthropic intervention. Renaissance Philanthropy believes that there is an opportunity to use TPPs in pursuit of a broader range of goals, including energy and climate, education, workforce development, and economic and social mobility.
The Play
Target product profiles can be a useful mechanism to address market failures and foster innovation
A TPP is a strategic document that summarizes the features of an innovation needed to address an unmet need. It outlines the desired characteristics of a target product by defining the intended use, target population(s), and other desired attributes, including safety and efficacy-related characteristics.
TPPs can serve varying purposes but their overarching goal is to help foster innovation. They can guide industry in research and development by providing a clear vision of a product’s objectives. TPPs communicate requirements but are not overly prescriptive in defining how to achieve the solution to the problem.
The TPP development process facilitates an open dialogue between the supply side—product developers, manufacturers, and innovators—and the demand side—which could include end-users and funders. If the product requires regulatory approval (e.g. vaccines, medical devices), involving organizations such as the FDA and the WHO may be important as well.
A well-defined TPP can provide a roadmap for product developers and help ensure that a product is ultimately broadly adopted and commercially viable.
What does the TPP development process look like?
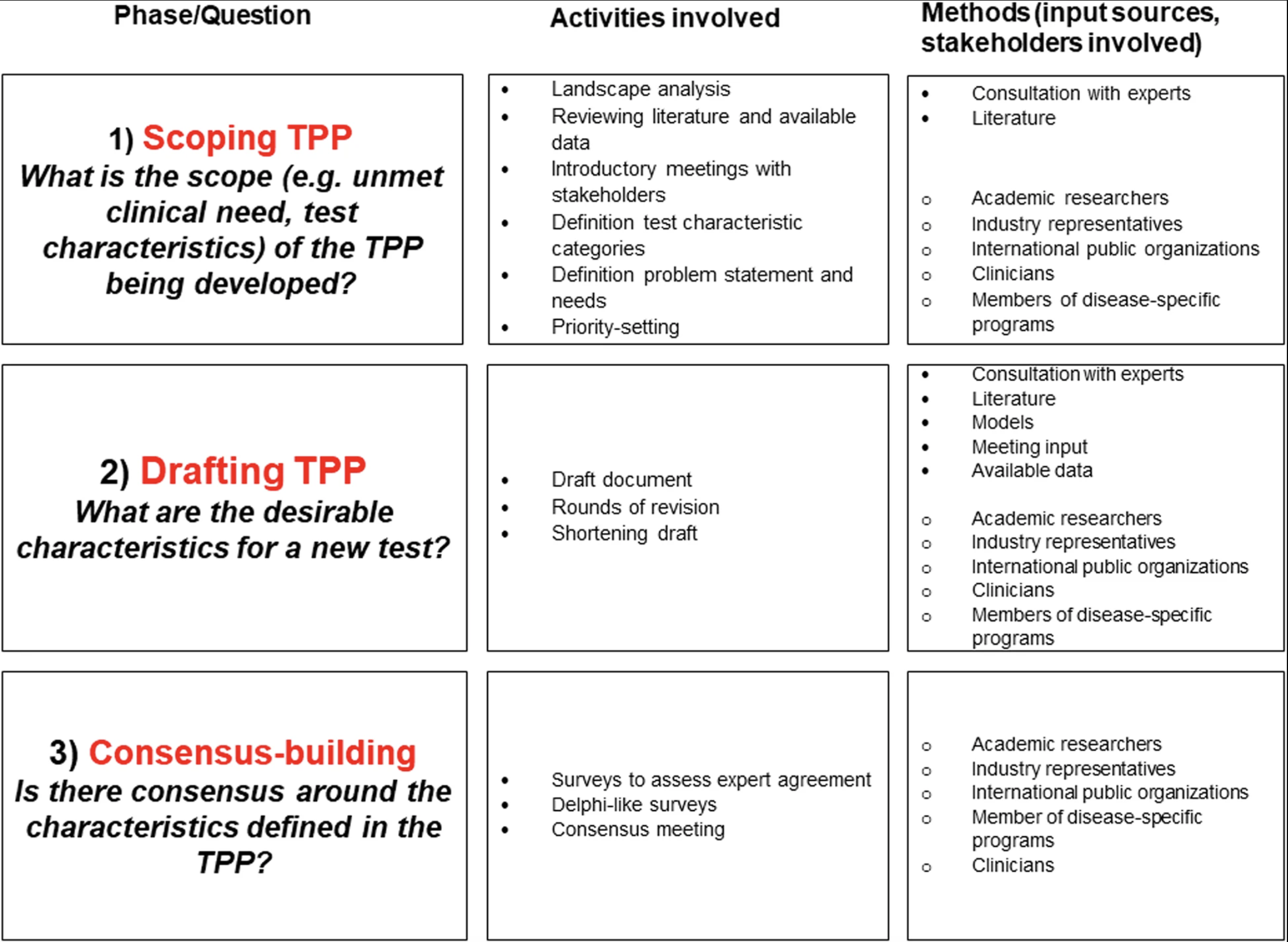
The development process itself is critical in fostering a valuable and stimulating discussion between stakeholders. A systematic review conducted by Cocco et al. surfaced three distinct phases for TPP development: scoping, drafting, and consensus-building.
An overview of the activities and methods involved in TPP development | By Cocco et al.
Step 1. Identify key stakeholders for initial draft development:
Proposed steps to design a TPP:
A scoping exercise may be used to provide an overview of the problem, define the unmet need and address the limitations associated with existing technologies. This can include a review of published literature, completion of a ‘landscape analysis’ or interviews with key stakeholders. Establishing a working group of key stakeholders and experts upfront can be instrumental in the ultimate success and adoption of the TPP.
Step 2. Hold a kick-off event to publicize TPP:
Once the initial, high-level draft TPP has been developed, convene leading experts to introduce the TPP process to a broader audience and gain buy-in early on. The event can include representatives from both industry and end-users to discuss challenges and opportunities for innovation.
Step 3. Distribute a survey:
Engage key stakeholders to determine alignment on minimal and optimal characteristics. Ask respondents to provide a statement on their level of agreement with each of the proposed product requirements. Agreement can then be scored on a Likert scale ranging from 1 to 5. Consensus for the survey characteristics should be pre-defined. For example, a group could aim to have more than 50% of respondents provide a score of at least 4.
Step 4. Hold a consensus meeting:
Upon completion of the survey, consensus should be reached on the final TPP. Hold an in-person or virtual consensus meeting gathering stakeholders to engage group discussion. At the consensus meeting, the focus can be directed towards areas of disagreement. If consensus is not achieved, this should be noted in the final published report.
Step 5. Publish a TPP report:
Engage key stakeholders to determine alignment on minimal and optimal characteristics. Ask respondents to provide a statement on their level of agreement with each of the proposed product requirements. Agreement can then be scored on a Likert scale ranging from 1 to 5. Consensus for the survey characteristics should be pre-defined. For example, a group could aim to have more than 50% of respondents provide a score of at least 4.
Figure depicting a high-level overview of the process to develop a TPP | By Becca Kirby
What characteristics should be included in a TPP?
Typically, TPPs address and define the “minimum” requirements, which refer to the lowest acceptable requirements, as well as “optimal” performance characteristics, which refer to the ideal or best case-scenario targets that products should aim to achieve. The goal of the TPP development process should be to ensure that these criteria are both desirable and realistically achievable.
Performance characteristics and product requirements will vary depending on the purpose of the TPP, but examples include:
Target Population
Intended Use
Target Setting
Technical Characteristics
Legal Characteristics, such as regulatory requirements
Manufacturing
Power Requirements, such as the need for a battery
Price. [This is particularly relevant if the product needs to be affordable for low-income countries.]
Creating a TPP is a collaborative exercise. Consensus-based TPP processes that involve a wide range of stakeholders can facilitate increased buy-in through open dialogue. Specifically, an open dialogue between end-users and product developers can capture trade-offs and allow stakeholders who may otherwise have limited interaction to learn from each other.
Key stakeholders might include representatives from the following groups: researchers, industry, international public organizations, advocacy groups and associations, policy makers, laboratory experts, technical/funding agencies, implementers, modelers, economists, donors, and market experts. While an independent group may take on the initial task of developing a TPP, collaborating with larger organizations and partners can prove beneficial.
Having representation from industry—including established manufacturers, researchers, and early-stage innovators—can prove invaluable in facilitating a healthy and productive dialogue. These representatives can bring an important perspective to the discussion which can help to ensure that the benefits are appropriately demonstrated so that the innovation will ultimately be commercially viable.
Who should be involved?
There are some risks associated with the use of TPPs, including:
Locking in to a fixed set of criteria, and not being responsive to new scientific findings, technological progress, developments in the market, or changes in policy.
Scope creep, adding too many requirements.
Setting unrealistic goals in terms of price and performance.
Failing to identify the need for market segmentation and trying to meet the needs of diverse customers with a single product.
Risks
Case Studies
TPPs have traditionally been used in healthcare in instances where market intervention is necessary to drive innovation, but there is also broader applicability across other industries.
Food & Drug Administration
In the United States, Target Product Profiles (TPPs) were initially used by the Food & Drug Administration (FDA) as a tool to facilitate communication between the pharmaceutical industry, the FDA, and other stakeholders outside of the industry to aid in the new drug development pipeline. Guidance issued by the FDA provides an overview of the purpose and attributes of TPPs, and which requirements for a new drug should be included.
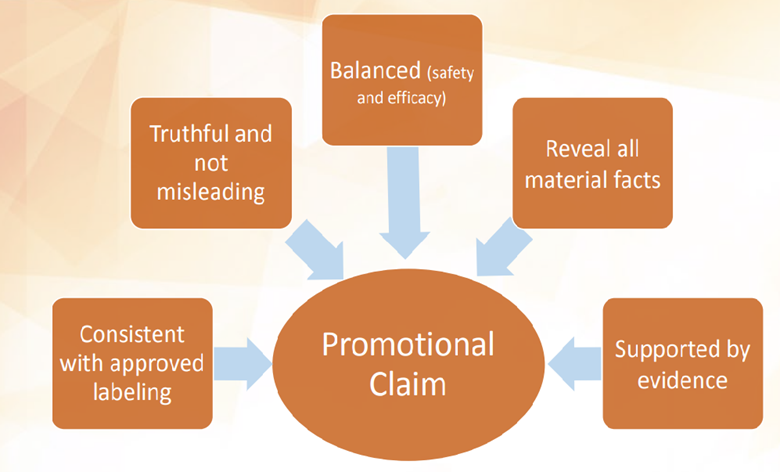
Traditionally, TPPs were used as a ”format for a summary of a drug development program described in terms of labeling concepts.” By focusing on the ”end game,” TPPs would effectively guide the design, conduct, and analysis of trials as well as ensure adequate collection of any additional information required to support labeling. Since specific needs, including labeling claims, are stated at the beginning of development, they serve to guide the design, conduct and analysis of preclinical and clinical trials, helping to guide and streamline discussions between manufacturers and regulators at all stages of product development. The process highlighted that an open, efficient dialogue could minimize the risk of late-stage drug development failures, increase the probability that optimal safety and efficacy data are available in a timely manner, and often decrease the total time to development.
The FDA’s TPP integrates general principles of promotion into a clinical development plan | Food & Drug Administration
Biomedical Advanced Research and Development Authority (BARDA)
Biomedical Advanced Research and Development Authority (BARDA), which promotes the advanced development of medical countermeasures to protect Americans, utilizes TPPs to provide a clear set of aspirational targets that can help focus and guide research and development activities. These TPPs provide structure for the scientific, technical, and clinical information required to achieve a desired outcome and provide stakeholders with a clear vision of the product objectives in order to help guide research and development decisions. Ideally, the BARDA TPPs will lead to products that will be approved by the FDA.
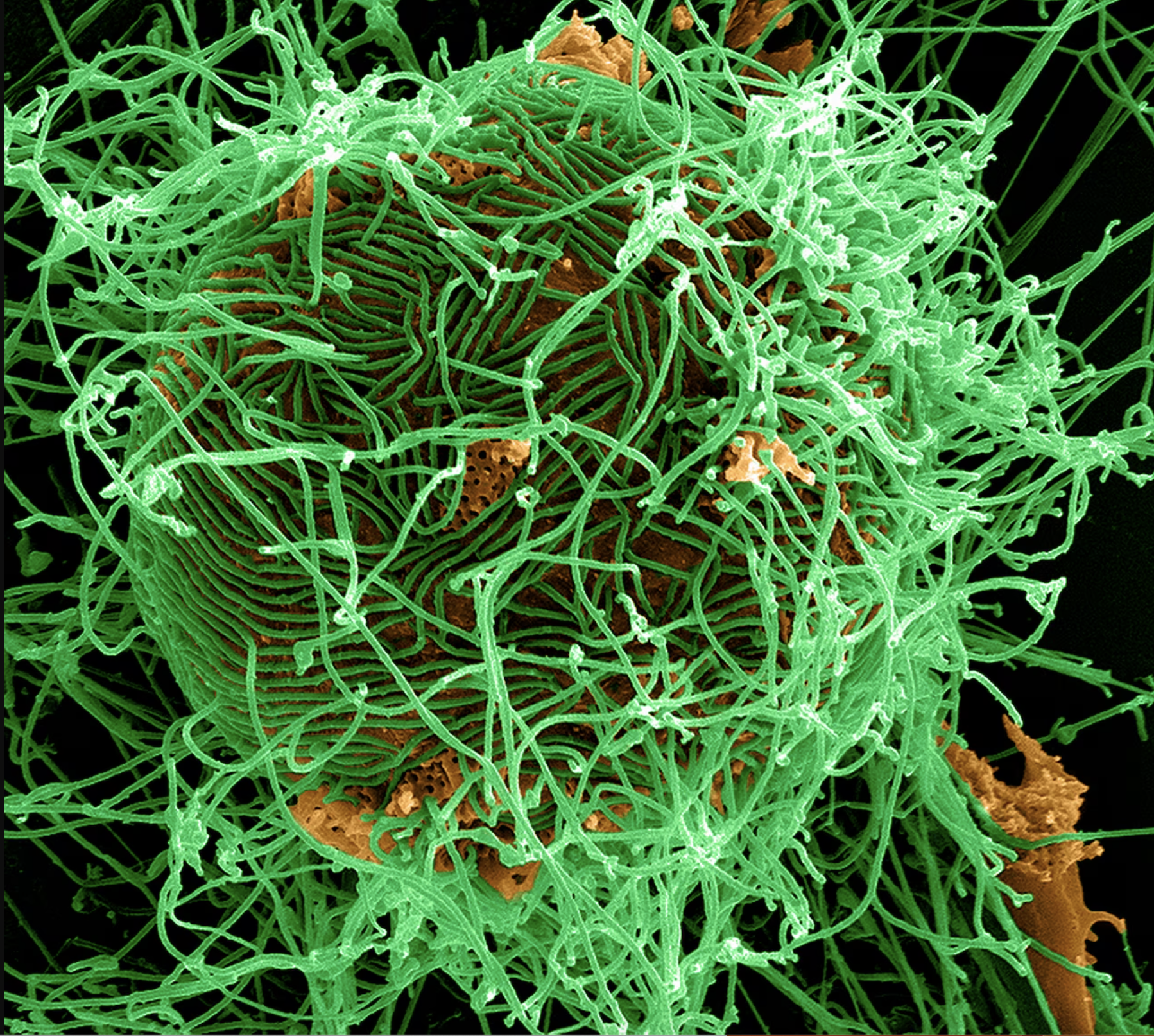
BARDA developed a TPP for a technology based on metagenomic next-generation sequencing that could allow us to detect novel pathogens, and help prevent the next pandemic. It has also published TPPs for vaccines, including a filovirus vaccine, to protect the American public from diseases such as the Ebola virus and Marburg virus. An effective filovirus vaccine would increase domestic preparedness for a potential Ebola outbreak, and it would allow the U.S. to help respond to a global health filovirus emergency.
BARDA’s TPP could lead to the creation of vaccinations against the Ebola and Marburg viruses. There are currently no licensed vaccines against these diseases. | National Geographic
International Agencies
Internationally, the WHO and other non-governmental organizations, including UNICEF, FIND, and PATH, utilize TPPs to help communicate requirements for needed innovations and to guide new product research and development. TPPs can be useful as a planning tool both for industry and regulators, and can help researchers, developers, and manufacturers design products for specific contexts that meet the needs of end-users.
In April of 2020, the WHO released a TPP for a COVID-19 vaccination for anyone at high risk of getting severely ill from the virus. The TPP outlines the WHO’s priorities for the vaccine’s development, including modeling vaccines that have different efficacy profiles, different modes of administration, and that could be utilized at different stages of the epidemic. The TPP for the COVID-19 vaccine has since been updated to reflect the information about, and availability of, COVID-19 vaccinations.
The WHO’s TPP guided the successful development of the COVID-19 vaccine, which was then administered in clinics across the country | Penn Medicine
This resource was adapted based on the playbook developed by Becca Kirby, Sustainability and Social Impact Program Research Assistant Professor and Lecturer at the Kellogg School of Management, Northwestern University. Access the original playbook and additional references here.
Resources
A call to bridge the diagnostic gap: diagnostic solutions for neonatal sepsis in low- and middle-income countries (BMJ Global Health)
BARDA Target Product Profiles (TPP) (BARDA)
Guidance for Industry and Review Staff Target Product Profile — A Strategic Development Process Tool (FDA)
Target product profiles (FIND)
Target product profiles (WHO)
Target Product Profiles for medical tests: a systematic review of current methods (BMC Medicine)
Target product profiles for neonatal care devices: systematic development and outcomes with NEST360 and UNICEF (BMC Pediatrics)
The target product profile as a tool for regulatory communication: advantageous but underused (National Library of Medicine)
TPP Consensus Meeting Template Example (Becca Kirby, Kellogg School of Management, Northwestern University)
TPP Overview (Becca Kirby, Kellogg School of Management, Northwestern University)
TPP report templates (Becca Kirby, Kellogg School of Management, Northwestern University)
TPP Survey Example (Northwestern University)
Use Cases: Sepsis Diagnostic — Infection Prevention and Control (UNICEF)